crystallising colours
2022
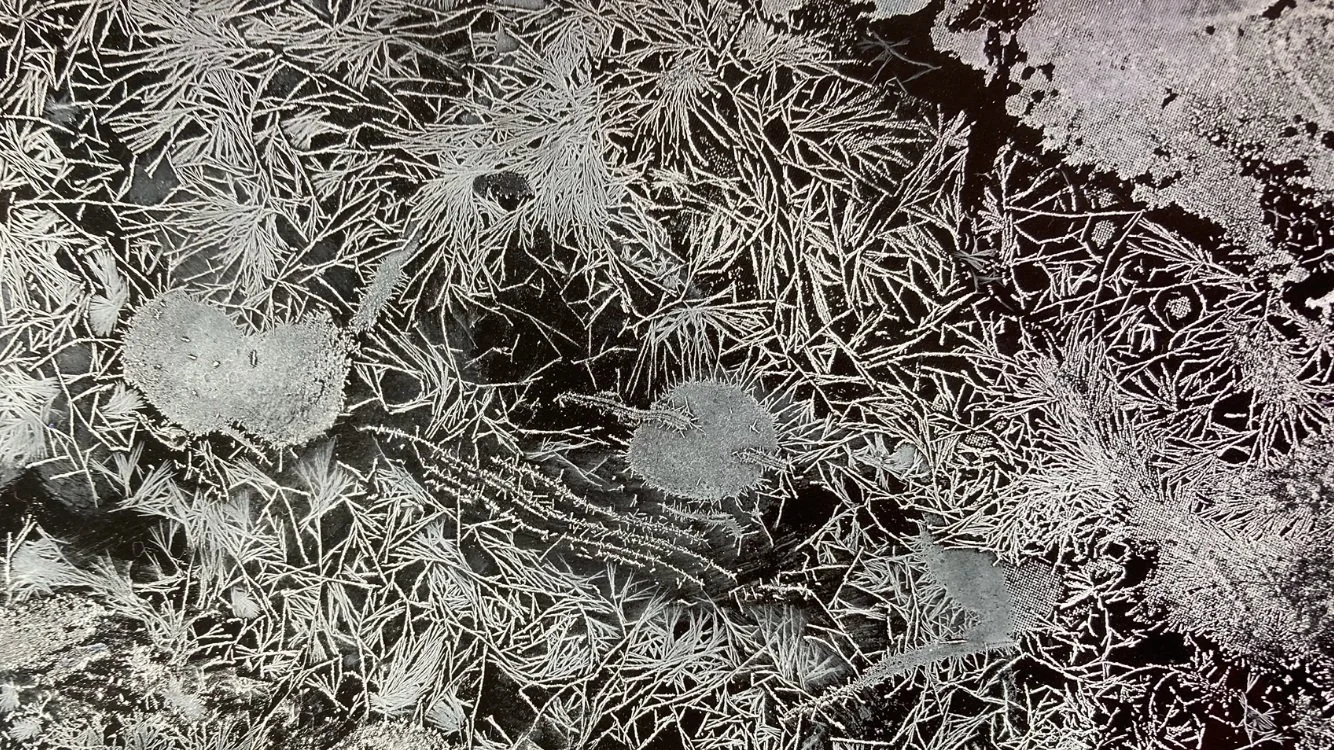
crystallising process
The process of crystallising involves a series of reactions in the space with temperature, minerals, opposing forces like magnets and also with sound.
In this process I have discovered that changing the crystallised structure of the colours or on the surface of the colours can produce really interesting visual results. Developing new tones, new textures and visual structures that add more depth to the surface and also add a new element to manipulate within the screen printing process and create resists with.
In a series of experiments I developed different ways to crystallise colours to get desired results. For example, in very cold temperatures I noticed that the structure would form much tighter bonds and smaller clustered of crystals. I also noticed that the speed at which the crystals would form at would also change the pattern and the natural direction they would grow in.
With this I played with manipulating the temperature, adding resists, using water to change the course of the process to see how to get different desired patterns across the fabric surface. Playing with a series of tools to manipulate the conditions to see what results I would get.
What is crystallisation
Crystallisation is a process by which a substance transitions from a disordered, liquid, or gaseous state to an ordered, solid state, forming a crystal structure. This natural phenomenon involves the arrangement of atoms, ions, or molecules in a highly organised and repeating pattern, creating a crystal lattice. The resulting solid, known as a crystal, typically exhibits well-defined geometric shapes and properties.
Key Points about Crystallisation:
Nucleation:
The process begins with nucleation, where individual particles (atoms, ions, or molecules) come together to form a small, stable cluster.
Growth:
Once nucleation occurs, the clusters continue to grow by the addition of more particles, gradually forming a larger and more structured solid.
Crystal Lattice:
The particles arrange themselves in a specific and repeating three-dimensional pattern, creating a crystal lattice. The arrangement is determined by the chemical composition and bonding forces within the substance.
Solubility:
Crystallization often involves the transition from a solution to a solid state. This can occur through processes such as cooling, evaporation, or changes in pressure, leading to a reduction in solubility and the precipitation of crystals.
Purity:
The purity of the substance undergoing crystallization is crucial. Impurities or foreign particles can disrupt the regular arrangement of the crystal lattice.
Temperature Control:
Temperature plays a critical role. Lowering the temperature typically promotes crystallisation by reducing the solubility of the substance in the solvent.
Seed Crystals:
Introducing seed crystals, which are small crystals of the same substance, can serve as nucleation sites and facilitate the growth of larger crystals.
Size and Shape:
The conditions under which crystallisation occurs, such as the rate of cooling or the presence of impurities, influence the size and shape of the resulting crystals.
In summary, crystallisation is a fundamental process that occurs in a wide range of natural and synthetic contexts, resulting in the formation of ordered and structured solids with specific properties. The conditions under which crystallisation occurs influence the characteristics of the resulting crystals.
Dye formula base
Working with the soluble of dye formula this was the base from where I would change the conditions.
The formation of crystals is influenced by various properties of substances. When certain conditions or parameters change, these properties can affect the crystallisation process.
Some examples of properties in substances that can undergo changes during crystallisation:
Solubility:
Change: The solubility of a substance in a particular solvent can change during the crystallization process.
Effect: As the solvent cools or evaporates, the solubility of the substance decreases, leading to the precipitation of crystals.
Temperature:
Change: The temperature of the substance or its surroundings can be a critical factor.
Effect: Lowering the temperature can lead to the formation of crystals as the solubility of many substances decreases with temperature.
Concentration:
Change: Altering the concentration of the solute in the solvent.
Effect: An increase in concentration, achieved through evaporation or other means, can lead to supersaturation and subsequent crystal formation.
Pressure:
Change: The pressure applied to the substance.
Effect: Changes in pressure can influence the solubility of certain substances, affecting the crystallization process.
Purity:
Change: The purity of the substance in terms of impurities or other components present.
Effect: Impurities may interfere with or inhibit crystallization, affecting the quality and characteristics of the crystals formed.
Agitation:
Change: The level of agitation or stirring during the crystallization process.
Effect: Agitation can impact crystal size and shape. Gentle stirring may encourage nucleation, while rapid agitation may hinder crystal growth.
pH Level:
Change: Altering the pH level of the solution.
Effect: Changes in acidity or alkalinity can affect the solubility of certain substances, influencing the crystallization process.
Presence of Seeds:
Change: Introducing seed crystals into the solution.
Effect: Seed crystals can serve as nucleation sites, initiating the crystallization process and influencing the size and structure of the crystals formed.
Rate of Cooling:
Change: Adjusting the rate at which the substance cools.
Effect: The rate of cooling can impact crystal size, with slower cooling often leading to larger crystals.
Molecular Structure:
Change: The molecular structure of the substance.
Effect: The arrangement of atoms and molecules in the substance influences how crystals form and the type of crystal lattice that is adopted.
Intermolecular Forces:
Change: The strength and nature of intermolecular forces.
Effect: Strong intermolecular forces, such as hydrogen bonding, can lead to the formation of well-defined crystals.
Understanding these properties and their potential changes is crucial for controlling and manipulating the crystallisation process. These are the factors I would adjust to achieve desired outcomes for the creation of crystals with specific characteristics.
Findings from using Dye formula as base for crystalistion
I found that when using natural colour dye forumlas like procion dye with manutex which is a binder made from seaweed. The crystallisation process was more organic.
Depending on what I added into the dyes also changed the outcome. For example if I added more resist salt it would create more of a crystal surface in the drying process due to the change in salinisation and density.
Also the use of soda ash at the end to fix the colours would also react with the surface of the fabric and subtly change the tones of the colours underneath due to the PH change. This proved to be very interesting as it started creating unique shades in different clusters adding a beautiful texture and depth to the fabric.
I also found that some colours which carried resists chalk from the screen would naturally have a more powdered crystal because it was being carried by the chalk which changed its concentration.
Those prints done with no resists would have really sharp crystal process because there was no other material intervention in the printing process and the soluble was more pure.
Heat, pressure and sound experiments
Crystallising substances using heat, pressure, and sound involves a unique and experimental process. While the details can vary depending on the specific substance and the intended outcome.
These experiments were not done in controlled spaces, they did have many variables but there was a general thread in the process and the outcomes which could allow me to do repeated experiments for similar outcomes.
Here is a vague outline of how I conducted the experiments:
Applying Heat:
Gradually applied heat to the substance using the chosen heat source. The heat should be controlled and maintained at a level that facilitates the crystallisation process without causing undesirable effects.
Applying Pressure:
If pressure is part of the process, apply it at the appropriate stage. This could involve pressing the substance between surfaces or using a pressurised system.
Introduce Sound:
Introduce sound waves to the substance using an ultrasonic device or another sound source. The frequency and intensity of the sound may influence the crystallisation process.
Monitor the Process:
Continuously monitor the substance to observe the crystallization process. Adjust heat, pressure, or sound parameters as needed based on the desired outcome.
Cooling Stage:
Once the crystallisation process is complete, allow the substance to cool. Controlled cooling is crucial to the formation of stable crystals.
Cooling may also change the bonds and the crystallisation process so pay attention in this point.
Inspect the Crystals:
After the substance has cooled, inspect the resulting crystals. The size, shape, and uniformity of the crystals can be influenced by the parameters you controlled during the process.
findings from heat, pressure and sound manipulations
Using the hairdryer and applying heat in certain areas, creating a trail and playing with the distance and surface area of the heat applied in certain areas manipulated the pattern of the how it would crystallise.
This was not a controlled experiment and there were many other factors involved that also could have changed the patterns. For example, I felt that music was a very big manipulation factor.
I found the music changed the vibration on the table and in the space which in turn produced different molecular bonds and changed how the atoms were vibrating which manifested in different ways.
Faster music created stronger more sharper bonds and forms. Softer music produces more subtle and dynamic shapes and forms. BPM was a big factor in the way the crystals form.
I also experiments with the amount of pressure used in the printing process to see if this made a difference. I found that areas that I applied more pressure to did create strong crystal bonds that formed little clusters.
These clustered formed very tightly together like domes, creating very beautiful movement and space interventions.
I found that this process was like looking into crystal rocks and seeing how they were formed over time, through pressure and minerals that made them specific transparencies, colours, weights, textures and shapes. I found this process deeply interesting and will continue to explore the possibilities of crystallisation as well as surface and formular maniuplation to continue producing interesting outcomes.
I would also like to take this to the next step and really work with the crystal forms to see how they can act as resits on a large scale to get bigger marks and surface area of crystals.